FAQs
Find answers to frequently asked questions about COVID-19, including how the virus is spread, risk factors for severe disease, and information on new monoclonal antibody treatments.
People aged 65 years and older and people of any age with serious underlying medical conditions may be at higher risk of severe illness from COVID-19.1 Preexisting medical conditions that increase the risk of serious illness include1,2:
- Chronic lung disease or moderate-to-severe asthma
- Cardiovascular disease, hypertension (high blood pressure), and serious heart conditions
- Diabetes
- Liver disease
- Cancer
- Chronic kidney disease and dialysis
- Obesity
- Immunosuppression (bone-marrow or organ transplantation, immune deficiencies, autoimmune diseases, poorly controlled HIV or AIDS, or long-term use of corticosteroids)
People who are at high risk of serious illness with COVID-19 should continue to wear face masks, practice social distancing, and use good hand hygiene to minimize their chance of contracting the virus that causes COVID-19.3
Other high-risk factors can be found on the CDC website at www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
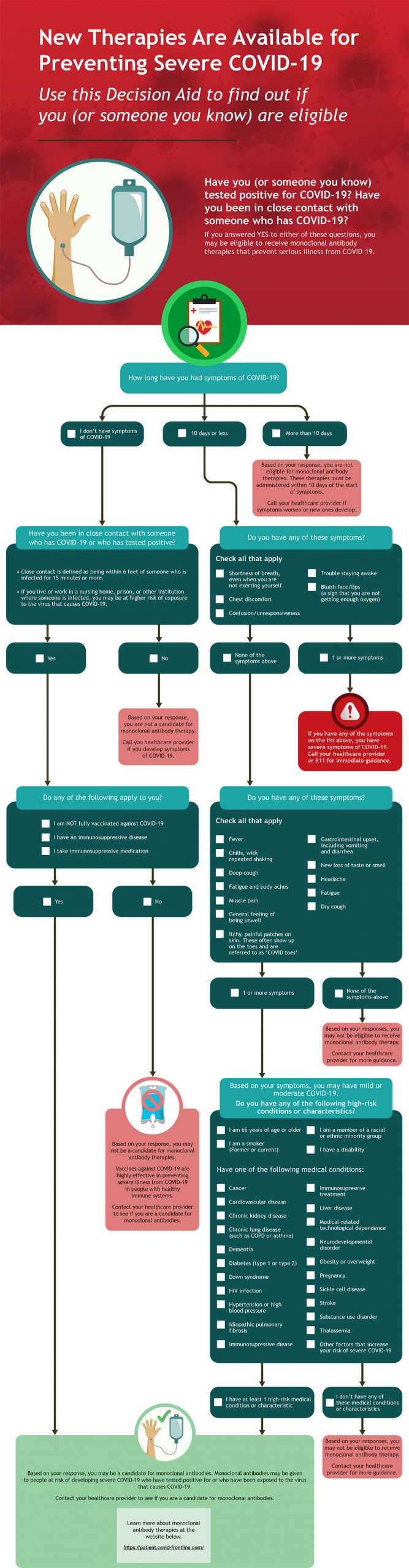
Antibodies are proteins created by your immune system to fight infection. Antibodies bind specifically to viruses and other microbes and target them for destruction by immune cells. Monoclonal antibodies have a similar structure to the antibodies created naturally by your immune system, but they are created in a laboratory. Monoclonal antibodies bind to the SARS-CoV-2 virus that causes COVID-19 and decrease the viral load (ie, number of viruses) in the body. Reducing the viral load means you may have milder symptoms and are less likely to be hospitalized with COVID-19.1,4
Monoclonal antibodies may help people who1,4:
- Have mild-to-moderate COVID-19, and
- Have had symptoms for 7 days or less, and
- Are at high risk for developing severe COVID-19
Monoclonal antibodies may also be used to prevent infection in patients who are exposed to the virus. If you have had close contact with someone who has COVID-19, monoclonal antibodies given soon after exposure can reduce your risk of getting sick. Speak with your healthcare provider as soon as possible to see if you are eligible for monoclonal antibody therapy.
Use the Monoclonal Antibody Eligibility Tool to find out if you (or someone you know) are a candidate for this treatment.
Symptoms could appear as soon as 2 days or as late as 14 days after exposure to the virus. The median time for symptoms to develop is about 5 days.1,2 If you believe you have been exposed to the virus that causes COVID-19, it is important to quarantine for 14 days to prevent the spread of the virus to others.3
It is estimated that on average people with COVID-19 first develop symptoms approximately 5 days after exposure to the virus. However, symptoms may develop between 2 and 14 days after exposure.1,2 One study found that people with COVID-19 were contagious approximately 2 to 3 days before symptom onset and were most infectious the day before symptoms appeared.
People infected with the new coronavirus can be contagious without symptoms.2 It is estimated that up to 50% of people infected with coronavirus remain asymptomatic. However, these people are still contagious. One study found that people with no symptoms were the source of 44% of diagnosed COVID-19 cases.
There are 3 available and recommended COVID-19 vaccines in the United States. All currently available vaccines are safe and effective in preventing COVID-19. You should get the first vaccine that is available to you. Do not wait for a specific brand.5
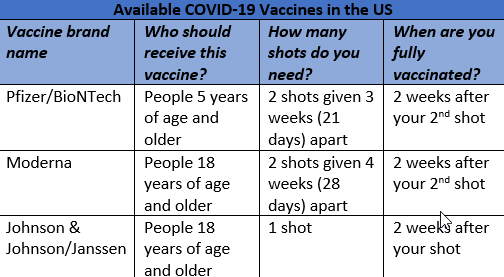
Protection against the virus that causes COVID-19 may decrease over time and the CDC recommends booster shots for everyone ages 5 years and older. A second booster shot is recommended for individuals 50 years and older and for certain immunocompromised people ages 12 years and older.
Vaccines for COVID-19 contain a small piece of the virus’ genetic material and do not contain the full virus. You cannot get COVID-19 from a vaccine.5
The genetic material in a vaccine allows our immune system to recognize the virus and produce long-lasting memory cells that fight future infections without ever getting the illness. It typically takes two weeks for your body to produce memory cells.5 Therefore, if you get infected with the virus that causes COVID-19 before or shortly after vaccination, you may get sick because your body did not have enough time to create memory cells against the virus.5
You may experience side effects after receiving a COVID-19 vaccine. Symptoms, such as fever, sore arm, chills, and fatigue, are normal and are signs the body is building immunity against the virus.5,7
Coronaviruses are a family of viruses that have crown-like spikes on their surface. The scientific name for the new coronavirus that emerged from China in December 2019 is SARS-CoV-2, which stands for severe acute respiratory syndrome coronavirus 2.1
COVID-19 is the disease caused by the new SARS-CoV-2 virus and stands for coronavirus disease 2019.
Some patients report long-lasting symptoms after recovering from COVID-19. Although these patients no longer have an active infection, they still experience complex symptoms caused by the virus weeks or sometime months after diagnosis. Persistent symptoms can happen to any one who has had COVID-19, even if their illness was mild or they had no symptoms.8
Commonly reported lingering health issues from COVID-19 include8:
- Fatigue
- Shortness of breath
- Anxiety
- Persistent cough
- Racing heartbeat
- “Brain fog” or problems with memory or concentration
- Depression
- Joint pain
- Muscle aches
- Dizziness
- Rashes
- Vision changes
- Loss of smell or taste
References
- National Institutes of Health (NIH). COVID-19 Treatment Guidelines Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Accessed June 5, 2022. https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf
- Centers for Disease Control and Prevention (CDC). Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Updated June 30, 2020. https://stacks.cdc.gov/view/cdc/89980
- World Health Organization (WHO). Q&A on coronaviruses (COVID-19) Last update May 13, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses
- Department of Health and Human Services (HHS). What Are Monoclonal Antibodies? Accessed June 5, 2022. https://combatcovid.hhs.gov/what-are-monoclonal-antibodies
- Centers for Disease Control and Prevention (CDC). Stay Up to Date with Your COVID-19 Vaccines. Updated May 4, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html
- Centers for Disease Control and Prevention (CDC). COVID-19 Vaccine Boosters. Updated May 24, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
- Centers for Disease Control and Prevention (CDC). What to Expect After Getting a COVID-19 Vaccine. Updated February 8, 2022. https://www.cdc.gov/coronavirus/2019-ncov/downloads/vaccines/324160-A-COVID-19_VaccinationPoster_WhatToExpect_LTR-6.24.pdf
- Centers for Disease Control and Prevention (CDC). Long COVID or Post-COVID conditions. Updated May 5, 2022. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
- Centers for Disease Control and Prevention (CDC). COVID-19 Vaccines for People who are Moderately or Severely Immunocompromised. Updated May 24, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html